If you’ve been around my practice or reading my blog for awhile, you may remember how sad I was over the loss of antibacterial soaps. I even wrote a blog post in 2016 about my favorites, and the FDA’s ruling to remove antibacterial soaps from the market that year.
On a trip to Sam’s Club a few months ago, I spotted Dial Antibacterial Soap. Now antibacterial soaps are showing up all over shelves at local stores and on the internet. Hooray!

As a review, in 2016 the FDA gave its final ruling on antibacterial soaps.
In summary, the FDA was concerned that widespread, consistent use of antibacterial soaps would induce bacterial resistance. However, no reliable population-based data showed that.
There was also some concern that, when used in extremes, there might be some systemic absorption as some of those ingredients had been detected in urine and breast milk.
In the end, the ruling stated that 17 chemicals previously used as antibacterial soaps could not be “Generally Regarded as Safe” because there was not sufficient evidence to demonstrate their efficacy and safety in the general population. Three antibacterial chemicals were still allowed to be used until they are further evaluated for evidence one way or the other.
Interestingly, the rule covered, “only OTC consumer antiseptic washes that are intended for use as either a hand wash or a body wash, and does not cover health care antiseptics, consumer antiseptic rubs, antiseptics identified as “first aid antiseptics,” or antiseptics used by the food industry.”
So, what is the primary ingredient it the new antibacterial soaps? Benzalkonium Chloride. According to Wikipedia, Benzalkonium Chloride was an organic salt classified as a quaternary ammonium compound. It has three main categories of use: as a biocide, a cationic surfactant, and as a phase transfer agent. It acts as a detergent, lysing cell membranes to and killing microorganisms, which makes it very effective as a preservative widely used in eye drops and cosmetic items.
Interestingly, there are some reports of it being irritating to the eyes and skin with continued use. The FDA now has challenged the American Cleaning Institute to prove the safety of benzalkonium chloride. At this time, the FDA has granted two extensions to the process to prove safety. For those who want more details, this is the most easily readable document I found on toxicology.
Should we use the new antibacterial soaps containing benzalkonium? Well, yes and no…. Yes, for me. I get exposed to a lot of germs each day, and MRSA is quite common in our community.
YES if you are in a “germy” environment. Using an antibacterial soap when exiting that environment is reasonable, particularly for the trunk and extremities.
NO for washing your face, ears, eye area, nose. No for everyday use unless you have had repeated skin bacterial infections. The safety data seems more questionable to me in that realm.
To further complicate things, there is increasing evidence of a positive role of bacteria on the skin. We will discuss the role of the SKIN BIOME in an upcoming blog post, so stay tuned!
If you know someone who may find this article helpful, please share it with them! Follow us on social media this week, and subscribe to our growing YouTube channel!
If you would like to receive these posts in your email inbox, Subscribe to our Site.
|












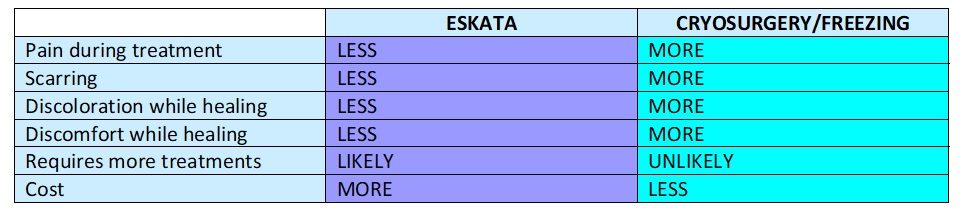
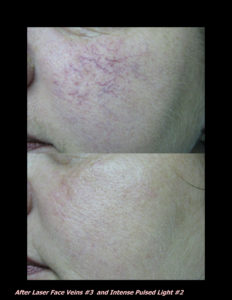
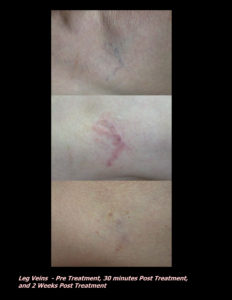
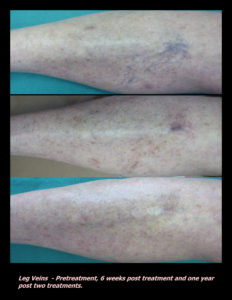
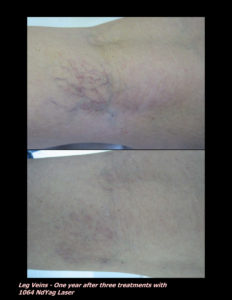
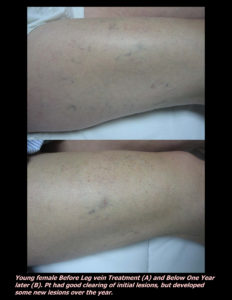
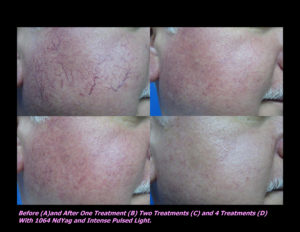
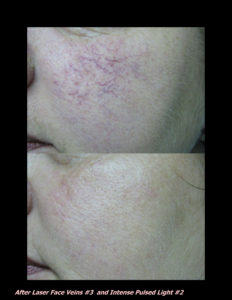
 Laser Vein Reduction Treatments
Laser Vein Reduction Treatments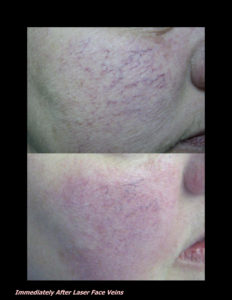



 DP Dermaceutical Products
DP Dermaceutical Products